The Ozempic lawsuit is drawing significant attention, focusing on claims that users weren’t properly warned about the drug’s potential risks. People who took Ozempic for both diabetes management and weight loss have reported severe side effects, prompting legal actions against the manufacturer.
These lawsuits are not only about the health impacts but also whether the company provided adequate warnings. In this article, we’ll explore the side effects that have sparked these Ozempic lawsuits, what claims are being made, and the compensation plaintiffs are seeking.
- Lawsuit status: The U.S. Judicial Panel on Multidistrict Litigation has consolidated over 1,300 federal lawsuits into a multidistrict litigation (MDL) in the Eastern District of Pennsylvania. These cases allege that Ozempic and similar GLP-1 receptor agonist drugs cause severe gastrointestinal injuries, including gastroparesis and intestinal blockages.
- Who can file: Individuals may be eligible to file a lawsuit if they used Ozempic as prescribed and suffered adverse health effects and experienced significant medical expenses, lost wages, or pain and suffering due to these health issues. Family members of deceased users who suffered from these conditions may also be eligible to file on their behalf.
- Estimated time: The timeline for resolving these lawsuits is uncertain and depends on various factors, including the complexity of individual cases and court schedules. MDLs can take several years to reach settlements or verdicts, especially as new cases continue to be filed.
- Settlement amounts: While no settlements have been finalized, potential compensation may vary based on factors such as injury severity, medical costs, and impact on quality of life. Estimates suggest that settlements could range from $70,000 to over $500,000, with severe cases potentially exceeding $1 million.
- Companies involved: The primary company involved is Novo Nordisk, the manufacturer of Ozempic. Some lawsuits also name Eli Lilly, the maker of similar medications like Mounjaro, alleging similar adverse effects.
What is the Ozempic Lawsuit?
Ozempic lawsuits began around 2021, as patients began claiming that the drug caused serious side effects that were not adequately communicated by the manufacturer. These initial cases primarily focused on issues such as gastrointestinal complications.
By 2022, the number of lawsuits increased significantly as more individuals using the drug, particularly for off-label purposes like weight loss, experienced severe health issues, leading to a surge in legal action.
As the Ozempic lawsuits continue to unfold, the case raises significant concerns about semaglutide medications like Ozempic, which is primarily prescribed to treat diabetes. Plaintiffs claim that Novo Nordisk, Ozempic’s manufacturer, failed to adequately address the risks associated with the drug.
Their claims particularly pointed out adverse effects such as severe vomiting, gallbladder disease, and bowel obstruction. These issues are at the heart of the complaints against the diabetes drug.
The legal actions, which have been consolidated into mass tort litigation, are being overseen by a judicial panel. This federal multidistrict litigation could potentially evolve into a class action lawsuit as more individuals affected by the drug’s side effects come forward.
Plaintiffs argue that Novo Nordisk failed to provide sufficient warnings about these risks on Ozempic’s warning label, despite evidence of serious adverse gastrointestinal events linked to the drug, including severe gastroparesis.
Additionally, some lawsuits allege that Novo Nordisk was negligent in addressing complications such as deep vein thrombosis that may arise from long-term use of the semaglutide drug. The complaint alleges that the drug manufacturers, including Novo Nordisk, prioritized profit over safety, which has led to numerous lawsuits being filed across the country. As these cases progress, plaintiff lawyers are examining whether drug manufacturers complied with regulations and provided adequate warning labels.
The outcome of these lawsuits, whether through individual settlements or rulings in court, will likely have significant implications for both Novo Nordisk and the broader category of pharmaceutical companies that produce similar semaglutide medications. As the litigation continues, individuals impacted by Ozempic’s side effects will be closely watching the legal developments in the eastern district and beyond.
Why Are People Suing Over Ozempic?
People are suing over Ozempic primarily due to the severe side effects they experienced, as well as the claim that Novo Nordisk failed to inform consumers of the risks they would expose themselves to.
While the drug was initially approved for diabetes management, its use for weight loss has led to unexpected and sometimes life-threatening complications.
The lawsuits focus on several critical issues:
- Severe Side Effects: Patients have reported debilitating conditions such as pancreatitis, bowel obstruction, severe vomiting, gallbladder problems, thyroid tumors, and stomach paralysis, which have led to hospitalizations and surgeries.
- Lack of Adequate Warnings: Plaintiffs claim that Novo Nordisk did not provide sufficient information about the risks associated with Ozempic, especially when used for weight loss.
- Negligence in Testing: Allegations also include that Novo Nordisk either failed to conduct proper safety testing or ignored concerning data from clinical trials, prioritizing profits over patient safety.
In these Ozempic lawsuits, the central argument is that Novo Nordisk knew, or should have known about the dangers of Ozempic but failed to communicate these risks effectively to both patients and healthcare providers.
Current Status of Ozempic Lawsuits (2024)
As of October 2024, the multidistrict litigation (MDL-3094) involving Ozempic, managed in the Eastern District of Pennsylvania, includes over 1,090 lawsuits against Novo Nordisk. Plaintiffs are reporting severe health complications such as gastroparesis and vision loss, with more cases expected as awareness of Ozempic’s side effects grows.
On October 21, 2024, Judge Gene E.K. Pratter denied a request by plaintiffs to obtain early access to specific Ozempic marketing documents. Plaintiffs argue that aggressive marketing of the drug overshadowed critical safety warnings, a focal point in the ongoing litigation. This ruling delays immediate discovery but leaves room for further arguments (Eastern District Court MDL).
With a significant increase in filings from August to September, Ozempic litigation is among the most rapidly expanding in the pharmaceutical sector. As media reports on these side effects, Novo Nordisk faces mounting pressure to address safety concerns and “failure to warn” claims, which are central to the MDL’s proceedings.
Timeline of Ozempic Lawsuit Filings
Below is a comprehensive timeline of the key events of the Ozempic side effects lawsuit filings:
2017: FDA Approval
Ozempic (semaglutide) was approved by the U.S. Food and Drug Administration (FDA) on December 5, 2017. The drug was initially approved to help manage blood sugar levels in adults with type 2 diabetes. At this point, no major concerns regarding side effects had been publicly raised.
2018–2020: Growing Popularity & Off-Label Use
Over the next few years, Ozempic became increasingly popular, not only for its approved use to treat diabetes but also for its off-label use as a weight loss aid.
August 2022: Patent Litigation and Gallbladder Risks
In August, seven patent cases involving Novo Nordisk were centralized in Delaware MDL to address generic competition for Ozempic. Around this time, JAMA Internal Medicine published findings linking Ozempic to higher risks of gallbladder disease, leading to initial gallbladder-related lawsuits.
August 2023: First Gastroparesis Lawsuit
On August 2, 2023, the first major gastroparesis lawsuit was filed, alleging severe gastrointestinal side effects. By September, the FDA updated Ozempic’s warning label to include risks of intestinal blockage and ileus.
December 2023: Precedent-Setting Rulings
A judge allowed most claims in a significant gastroparesis case to proceed while dismissing warranty claims. To manage the growing litigation, plaintiffs filed for further case consolidation in Louisiana.
January 2024: FDA Review of Adverse Events
The FDA reviewed adverse events reported with Ozempic, including hair loss and reports of suicidal thoughts (See CNN Health news), as these emerged in the FDA’s Adverse Event Reporting System database.
February 2024: MDL Established with 55 Cases
The JPML consolidated 55 lawsuits involving Ozempic into multidistrict litigation (MDL) in the Eastern District of Pennsylvania, expecting potential growth in cases regarding gastrointestinal side effects.
June 2024: Judge Marston’s Leadership and Bellwether Preparation
Judge Karen S. Marston took over the MDL, convening with attorneys to coordinate discovery and select cases for bellwether trials
July 2024: Vision Loss Study Spurs New Lawsuits
A JAMA Ophthalmology study linked semaglutide to nonarteritic anterior ischemic optic neuropathy (NAION), leading to lawsuits alleging vision loss and blindness from Ozempic use.
September 2024: Case Count Surges to 869
MDL cases increased from 346 in early August to 869 by September, reflecting a rapid expansion as more plaintiffs joined the litigation
For up-to-date case numbers and additional legal information, consult the U.S. Judicial Panel on Multidistrict Litigation and FDA resources.
Ozempic Side Effects
The lawsuit alleges that many plaintiffs claimed to have experienced severe health issues after taking the drug, and these side effects have led to significant physical, emotional, and financial consequences for patients and their families.
Below is an overview of the major side effects, including both common and more severe reactions.
- Nausea: One of the most commonly reported side effects of Ozempic is nausea. While nausea is often a manageable symptom, some patients report experiencing prolonged and severe nausea that interferes with their daily lives.
- Vomiting: Vomiting is another side effect associated with Ozempic. In some cases, patients have reported that they experienced frequent and severe vomiting after taking the drug, which led to dehydration and other complications.
- Pancreatitis: Pancreatitis is a serious and potentially life-threatening condition. Some patients who have used Ozempic claim that they developed pancreatitis as a result of taking the drug. Pancreatitis can cause severe abdominal pain, nausea, vomiting, and fever, and it often requires hospitalization.
- Gallbladder Issues: Several patients have reported developing gallbladder problems, including gallstones, after using Ozempic. Gallstones can cause severe pain and may require surgery to remove the gallbladder.
- Thyroid Tumors: One of the most concerning side effects of Ozempic is the development of thyroid tumors. Many lawsuits have been filed by individuals who developed thyroid tumors after taking Ozempic. They claim they were not adequately warned about this risk.
These side effects have significantly impacted the lives of the patients who experienced them. Now, let’s take a closer look at the most severe side effects associated with Ozempic, which have been central to many legal claims due to their serious impact on patient’s health and well-being.
While the common side effects are concerning, the severe side effects of Ozempic have garnered the most attention in the legal proceedings. These side effects, some of which have been life-threatening, include conditions that require extensive medical treatment and, in some cases, lead to permanent health damage.
Below is an in-depth look at the most severe side effects, their potential impact on patients’ health, and their role in the ongoing litigation.
Pancreatitis
One of the most serious side effects associated with Ozempic is pancreatitis, a condition that causes pancreas inflammation. The pancreas is essential for digestion and blood sugar regulation, and when it becomes inflamed, it can cause intense abdominal pain, nausea, vomiting, and fever. In severe cases, pancreatitis can lead to infection, tissue damage, and even life-threatening complications such as organ failure.
The FDA prescribing information specifically highlights pancreatitis as a known risk of GLP-1 receptor agonists like Ozempic, especially for individuals with a history of the condition.
Patients who have developed pancreatitis after using Ozempic often require hospitalization and may need surgery to address the inflammation. This condition can have long-term effects on a person’s health, including chronic pain and digestive issues. For some, the damage to the pancreas is irreversible, leading to permanent changes in their ability to digest food and regulate blood sugar levels, as detailed in MedlinePlus.
Many of the lawsuits filed against Novo Nordisk include claims from patients who developed pancreatitis after taking Ozempic. These plaintiffs argue that they were not properly warned about the risk of developing such a serious condition and that had they been informed, they would have reconsidered using the drug.
Patients using Ozempic for weight loss, a non-FDA-approved purpose, have expressed concerns. Many claim they were not informed about the risk of pancreatitis. This issue has become especially prominent among users seeking weight loss benefits.
Thyroid Tumors
Another major concern is the potential for developing thyroid tumors, a condition that has raised significant alarm in the medical community. Thyroid tumors can be benign (non-cancerous) or malignant (cancerous), but in both cases, they can pose a serious threat to a patient’s health.
Ozempic carries a boxed warning for thyroid C-cell tumors based on studies showing risks in animals, and this warning applies especially to those with a personal or family history of thyroid cancer. Some individuals who developed thyroid tumors after using Ozempic required surgery to remove them. Others needed radiation or chemotherapy to treat cancerous growths.
The connection between Ozempic and thyroid tumors has become a focal point of many lawsuits. Plaintiffs claim that they were not adequately informed about this risk, particularly when using the drug for an extended period.
While not every patient who takes Ozempic will develop thyroid tumors, those who do face the possibility of life-long monitoring and treatment. In the worst cases, malignant tumors may spread to other parts of the body, leading to more severe and life-threatening complications.
The development of thyroid tumors is a central issue in the Ozempic lawsuits because of the serious and potentially fatal consequences for patients. Legal complaints often focus on the failure to properly communicate the risk of tumors, especially when Ozempic is used off-label for weight loss.
Stomach Paralysis (Ozempic Gastroparesis Lawsuit)
Stomach paralysis, or gastroparesis, affects stomach muscles, causing them to stop functioning properly and leading to delayed digestion. Gastroparesis can cause symptoms like nausea, vomiting, severe abdominal pain, and bloating. In some cases, patients may be unable to digest food properly, leading to malnutrition and other serious health issues.
Stomach paralysis can be a debilitating condition that severely impacts a person’s quality of life. Patients with gastroparesis often struggle to eat, maintain a healthy weight, and stay hydrated. In some cases, individuals may need to rely on feeding tubes or other medical interventions to manage the condition.
Several patients who have developed stomach paralysis after taking Ozempic have filed lawsuits against Novo Nordisk, claiming that the company did not warn them about the risk of this condition.
These lawsuits, often referred to as Ozempic stomach paralysis lawsuits, argue that Novo Nordisk should have been more transparent. They claim the company failed to fully disclose the potential for developing gastroparesis, a condition with serious and long-lasting effects.
For those affected by stomach paralysis, the consequences are not only physical but also emotional and financial. Many patients have had to take time off work, undergo multiple medical procedures, and make significant changes to their diet and lifestyle.
Gallbladder Disease (Ozempic Gallbladder Lawsuit)
Gallbladder disease is another severe side effect reported by Ozempic users. Some patients have developed gallstones or other gallbladder issues after taking the drug. Gallstones can cause severe pain in the abdomen, and in some cases, surgery is required to remove the gallbladder altogether. For individuals who develop gallbladder disease, the condition can lead to long-term digestive issues.
Who Can File an Ozempic Lawsuit?
To qualify for a lawsuit, individuals typically need to show that they suffered from one or more of the severe side effects linked to the drug.
There are several key factors that determine whether you can file an Ozempic lawsuit:
- Experience of Severe Side Effects: If you have suffered from significant health issues like what is listed above after using Ozempic, you may be eligible to file a lawsuit. In some cases, the family members of individuals who died as a result of these side effects may also be able to file wrongful death claims.
- Inadequate Warnings: Many lawsuits are based on the claim that Novo Nordisk did not provide adequate warnings about the potential risks of Ozempic. If you were not informed of the side effects before taking the drug, this could form the basis of a legal claim.
- Off-Label Use: While Ozempic is approved for the treatment of type 2 diabetes, many individuals have used the drug off-label for weight loss. If you were prescribed Ozempic for weight loss and suffered severe side effects, you may have grounds for a lawsuit.
The first step in filing a lawsuit is to consult with a legal professional who specializes in pharmaceutical litigation. Ozempic attorneys can help determine whether you have a valid claim and guide you through the legal process.
Take Note: Working with experienced Ozempic lawyers is essential for ensuring your case is handled properly. This gives you the best chance of recovering compensation for your injuries.
Ozempic Class Action Lawsuits
The Ozempic class action lawsuits focus on claims that the drug caused severe side effects, including the diseases mentioned above. While these lawsuits are ongoing and no final settlements have been reached, claimants hope for compensation that could cover medical costs, pain and suffering, and lost wages. Legal teams are actively working on cases, which may lead to open settlements once initial test cases (bellwether trials) help determine likely outcomes.
Individuals affected by severe side effects may be eligible to join these class actions if they can demonstrate that their health issues are linked to Ozempic and that the warnings provided were inadequate. As of now, those who qualify can consult resources like Legal Reader and NST Law for further information on participating in future open settlements. Woods & Woods, Veterans Lawyer
Now, let’s learn the differences between individual and class action lawsuits to help you understand your legal options more clearly.
Individual vs. Class Action Lawsuits
For those considering legal action over Ozempic side effects, both individual lawsuits and class actions offer specific benefits depending on the type and severity of injuries.
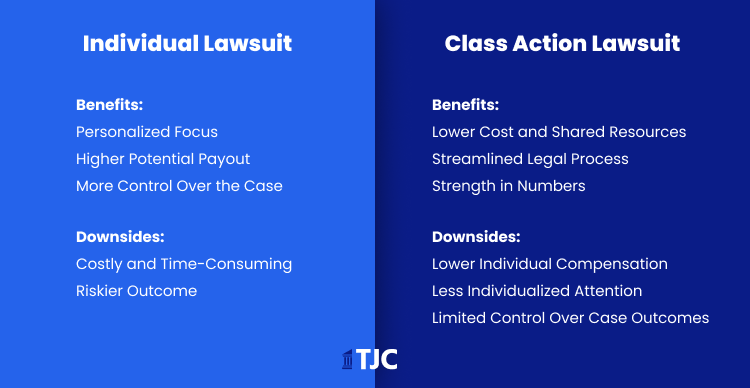
Individual Lawsuits
Plaintiffs with severe or unique injuries, like life-altering gastroparesis or extensive medical needs from pancreatitis, may benefit from filing an individual lawsuit. This approach allows for a tailored case, focusing on the specific impact of Ozempic on the individual’s health and financial losses.
These cases can potentially lead to higher settlements, particularly for those with serious, unique conditions that require more extensive evidence and compensation for personal damages
Class Action (or MDL)
For those who have experienced more common side effects, joining the existing Ozempic multidistrict litigation (MDL) offers a more efficient path. This option reduces legal expenses by pooling resources and standardizing evidence, which is ideal for claimants who want a streamlined process. Settlements may be lower in class actions but are often quicker, benefiting plaintiffs with moderate health impacts or typical complications from Ozempic
Choosing the right approach depends on the individual’s experience with Ozempic and their need for either a personalized case or a more straightforward, collective action. Consulting a legal expert helps clarify which path best suits the plaintiff’s specific circumstances.
How Much Could the Ozempic Lawsuit Payout Be?
Legal experts estimate that settlement amounts could range from $400,000 to $700,000 for plaintiffs who suffered the most serious injuries, such as gallbladder-related complications. These figures are based on previous pharmaceutical cases and the extent of harm caused by the drug. Each case will vary, but with the right legal representation, plaintiffs could receive substantial compensation for their injuries.
It’s important to note that settlements can differ based on individual circumstances. For example, those with long-term or life-altering side effects may receive higher compensation than those with milder cases.
Remember: As the lawsuits progress, these estimates may become more precise, especially as more cases are settled or go to trial.
Factors That Affect Settlement Amounts
Several key factors will influence how much a plaintiff could receive in an Ozempic lawsuit:
- Severity of Injury: The more severe the side effects, the higher the potential payout. People who suffered life-threatening or permanent damage, or long-term health issues, could receive larger settlements.
- Medical Costs: Compensation often reflects the total medical expenses incurred by the plaintiff. This includes hospital bills, surgeries, medication, and long-term treatments. Higher medical costs typically result in higher settlements.
- Lost Wages and Income: If a plaintiff missed work due to Ozempic-related injuries, they could be compensated for lost wages. In cases of permanent disability, future earning capacity could also be included in the settlement.
- Number of Plaintiffs: In class action lawsuits, the total settlement is divided among all the plaintiffs. The more people involved, the smaller the individual payout. In contrast, individual lawsuits may lead to larger compensation for the plaintiff, as they aren’t sharing the settlement with others.
- Pain and Suffering: Emotional and physical distress play a role in determining settlement amounts. Plaintiffs experiencing significant pain, mental anguish, or a reduced quality of life may be eligible for higher compensation.
Legal Grounds for Ozempic Lawsuits
The Ozempic lawsuits are built on several core legal claims, each holding the drug’s manufacturer accountable for the alleged harm caused. The most prominent claims include failure to warn, product liability, and medical negligence.
Failure to warn is central to many lawsuits, with plaintiffs alleging that users weren’t sufficiently informed about the risks. When patients or doctors aren’t made fully aware of the potential dangers, companies can be held legally responsible for any resulting harm.
Medical negligence also plays a role in some cases, where it’s argued that the manufacturer did not take proper steps to ensure the safety of its users. This negligence claim suggests that better testing, closer monitoring, or stronger warnings could have prevented injuries.
Product Liability and Pharmaceutical Lawsuits
Product liability law plays a central role in the lawsuits against Ozempic. In general, product liability holds manufacturers responsible when their products cause harm due to defects or inadequate safety measures. In pharmaceutical cases, this includes ensuring the drug is safe for its intended use and that all risks are clearly communicated to both doctors and patients. If a company fails in either of these duties, they may be held liable for any resulting harm.
For example, in the case of the diabetes drug Ozempic, the legal argument centers on whether the manufacturer provided sufficient warnings about potential risks, especially for patients using the drug off-label for weight loss.
Product liability claims in these cases often focus on inadequate labeling, where plaintiffs argue that if they had been better informed of the risks, they could have made safer decisions regarding their treatment.
How to File an Ozempic Lawsuit
If you’ve been affected by Ozempic and are considering legal action, here’s a step-by-step guide to filing a lawsuit:
- Consult with an Ozempic Lawyer: Start by finding a lawyer who specializes in pharmaceutical cases. A reputable law firm can assess the strength of your claim and guide you through the legal process.
- Gather Medical Records: Your medical records are critical evidence. These records should show that you were prescribed Ozempic and detail the side effects or injuries you’ve suffered, such as those cited in the Ozempic stomach paralysis lawsuit.
- File the Complaint: Your lawyer will draft and file a formal complaint with the court. This step officially gets your Ozempic lawsuit filed and outlines your legal claims and the damages you’re seeking.
- Prepare for Discovery: This phase involves gathering evidence from both sides, including medical documentation, internal company communications, and expert testimony.
- Settlement Negotiations or Trial: Many lawsuits end in settlements, where the company agrees to pay damages without going to trial. If no settlement is reached, the case proceeds to court, where a jury or judge will decide the outcome.
Similar Lawsuits Against Other Pharmaceutical Companies
Several pharmaceutical companies have faced lawsuits over diabetes medications and weight loss drugs, similar to the Ozempic class action lawsuit. For example, Januvia and Byetta (Merck and Bristol-Myers Squibb) were sued for allegedly causing pancreatitis, with claims focusing on failure to warn about the risks, just like in the Ozempic case.
In the weight loss drug category, Alli and Xenical (GlaxoSmithKline) were linked to liver damage, leading to lawsuits over inadequate warnings. Invokana (Johnson & Johnson), another diabetes drug, faced legal action for severe side effects, such as kidney failure. These cases mirror concerns in the Ozempic litigation, where plaintiffs argue they weren’t warned about risks associated with similar drugs.
What to Expect During the Lawsuit Process
Filing a lawsuit can be a long and complex process. Here’s a breakdown of what you can typically expect:
- Discovery: Both sides gather evidence, including medical records, expert opinions, and internal documents from the drug company. This stage helps both sides understand the strengths and weaknesses of their case.
- Pre-trial Motions: Before trial, lawyers may file motions to dismiss the case or exclude certain evidence. These motions can significantly impact the course of the lawsuit.
- Settlement Negotiations: Many lawsuits end in settlement negotiations, where the defendant agrees to pay damages to avoid the uncertainty of a trial. If a settlement offer is accepted, the case is resolved without going to court.
- Trial: If no settlement is reached, the case proceeds to trial, where both sides present their arguments. The outcome is decided by a judge or jury.
Ozempic Lawsuit Settlement Amounts
Ozempic lawsuits are still ongoing, with no finalized settlements reported. However, legal experts anticipate that settlement amounts could vary significantly based on individual circumstances. Estimates suggest that settlements for severe injuries, such as gastroparesis (stomach paralysis), may range from $400,000 to $700,000.
Several factors influence these potential settlement amounts:
- Severity of Injuries: More serious health complications typically lead to higher compensation;
- Medical Expenses: Costs incurred for treatments, hospitalizations, and ongoing care are considered;
- Lost Wages: Compensation for income lost due to the inability to work resulting from health issues;
- Pain and Suffering: Non-economic damages addressing physical and emotional distress;
- Punitive Damages: In cases of egregious conduct by the manufacturer, additional amounts may be awarded to deter future misconduct.
It’s important to note that these figures are speculative, as no settlements have been finalized. Most commonly, the cases may settle out of court, with the company agreeing to pay damages to the plaintiffs without admitting liability.
How Ozempic Manufacturer Novo Nordisk is Responding
The manufacturer of Ozempic has faced significant legal challenges as more lawsuits have been filed. So far, the company has publicly maintained that their product is safe when used as directed. However, with the ongoing Ozempic MDL and numerous individual lawsuits, the company is facing increasing pressure to defend its product in court.
Company Response to Allegations
Novo Nordisk asserts that Ozempic was rigorously tested and approved by the FDA, with labeling they believe adequately informs patients and doctors about potential risks, including serious gastrointestinal side effects. The company maintains that their warnings are clear and in compliance with FDA standards, defending both their labeling and clinical testing practices. Novo Nordisk official response
However, plaintiffs argue that the warnings weren’t thorough enough, especially for off-label use like weight loss. As part of its strategy, Novo Nordisk is also pursuing legal action against unauthorized compounded versions of semaglutide, highlighting concerns over safety risks posed by these unapproved alternatives.
FDA’s Role in the Ozempic Lawsuit
The FDA plays a crucial role in the Ozempic lawsuits, as the agency approved the drug for treating type 2 diabetes. Part of the plaintiffs’ claims may focus on whether the FDA’s approval process fully considered the risks, especially for off-label uses.
While the FDA does require strict testing before approval, some lawsuits argue that post-market surveillance—when the drug is already on the market—was insufficient. If the FDA issues additional warnings or recalls for Ozempic, it could further impact the lawsuits. At this stage, the FDA has not recalled the drug but continues to monitor reports of adverse side effects.
The Role of Semaglutide Drugs in Diabetes Management and Weight Loss
Semaglutide, the active ingredient in Ozempic, was originally developed to help manage blood sugar in patients with type 2 diabetes. It works by mimicking the body’s natural hormone that regulates insulin and blood sugar levels.
However, its off-label use for weight loss soon gained popularity. Many patients turned to the drug for its weight-loss effects, which became a widespread, though not FDA-approved, use. The unintended consequences of this shift have been at the core of many lawsuits.
While semaglutide drugs like Ozempic showed promise in helping people manage diabetes, their use among non-diabetic patients raised concerns. For those patients, it wasn’t clear what long-term side effects could emerge.
This is one reason plaintiffs have been so vocal in their lawsuits—many feel that they were misinformed about the potential risks of using Ozempic outside of its intended purpose. In hindsight, this off-label use could have contributed to the rise in severe side effects like gallbladder disease, pancreatitis, and gastroparesis.
The Impact of Off-Label Use and FDA Oversight
Ozempic’s growing use as a weight-loss medication highlights a key issue in pharmaceutical regulation: off-label use. Once a drug is approved by the FDA, doctors have the authority to prescribe it for conditions other than those it was intended for.
Though this can sometimes lead to positive outcomes, it also comes with risks. In Ozempic’s case, it was approved for treating diabetes, but its use as a weight-loss aid was never fully studied for its safety in that context.
As more patients turned to Ozempic for weight loss, reports of adverse side effects grew. Patients began experiencing serious complications, and questions arose about whether Novo Nordisk and other pharmaceutical companies were transparent enough about these risks.
The FDA has strict requirements for warning labels, but critics argue that the dangers of using semaglutide drugs for weight loss were not made clear enough to patients and healthcare providers. This has become a major focal point in the lawsuits against Novo Nordisk, where plaintiffs claim they were not warned about the possibility of developing severe health problems like gallbladder disease or bowel obstruction.
How Medical and Legal Communities Are Responding
The rise of lawsuits against drug manufacturers Novo Nordisk have drawn significant attention from both the medical and legal communities. Doctors are now more cautious about prescribing semaglutide medications for weight loss, particularly in patients without diabetes. Some have called for tighter regulations around the off-label use of drugs like Ozempic to prevent similar issues in the future.
Meanwhile, plaintiff lawyers are busy building cases that argue Novo Nordisk could have done more to inform the public about the risks of using Ozempic for non-diabetes purposes. They’re focusing heavily on how the company marketed the drug and whether the warning labels were adequate.
Legal experts expect more cases to be filed as public awareness grows, especially as patients who initially used the drug for weight loss may not have realized the full extent of the risks.
In terms of judicial proceedings, federal multidistrict litigation (MDL) has helped consolidate these cases, making the process more efficient for both plaintiffs and the courts. The litigation is still ongoing, but experts anticipate that Novo Nordisk may eventually opt for settlements to avoid lengthy and costly trials.
Looking Ahead: What’s Next for Novo Nordisk?
As the lawsuits against Novo Nordisk continue, the company faces a difficult road ahead. The outcome of these cases will not only affect the company’s bottom line but could also impact how other pharmaceutical companies handle drug marketing and patient safety. The warning labels must be clear and informative for consumers.
Novo Nordisk has maintained that Ozempic is safe when used according to its intended purpose, but plaintiffs argue that more could have been done to warn them about the risks, especially for off-label uses.
Moving forward, it’s possible that the company will face stricter regulations and may even have to revise the warning labels on Ozempic and other semaglutide drugs. For patients affected by the severe side effects, these lawsuits represent a chance to hold the company accountable for the harm they believe they have suffered.
In addition to the legal implications, the medical community will likely continue to monitor the long-term effects of Ozempic and other semaglutide drugs. As more data becomes available, there could be changes in how these drugs are prescribed, particularly for off-label uses.
For patients considering using Ozempic or similar medications in the future, it’s crucial to stay informed and consult closely with healthcare providers about the potential risks and benefits. The outcome of these lawsuits may not only provide compensation to those affected but also bring about important changes in the way diabetes and weight-loss medications are used.
Conclusion
The Ozempic lawsuits have major implications for consumers, potential claimants, drug manufacturers, and the pharmaceutical industry as a whole. For consumers, they emphasize the importance of knowing the risks before using medications, particularly for off-label purposes. Potential claimants now have a clear route to seek compensation for injuries caused by the drug.
For the pharmaceutical industry, these lawsuits stress the need for clear warnings and thorough testing. Failing to do so can lead to legal challenges and damaged reputations. For readers, these cases are a reminder to always ask questions about the medications you’re prescribed and to be informed about potential risks.
Beyond the immediate legal implications, these lawsuits may also influence how future drugs are marketed and regulated. As pharmaceutical companies navigate this growing wave of litigation, there could be stronger oversight from regulatory bodies like the FDA, pushing for more rigorous post-market studies and clearer communication of side effects.
This serves as a wake-up call for the industry, highlighting the significant costs—both financial and reputational—associated with inadequate consumer safety measures. For consumers, this situation reinforces the importance of advocating for transparency and accountability in the healthcare system.